SHINE’s Chrysalis production building, under construction in October 2022. (Photo: SHINE)
The Nuclear Regulatory Commission has issued the final supplemental environmental impact statement (EIS) for SHINE Technology’s application for a license to operate a medical isotope production facility in Janesville, Wis.
SHINE’s isotope production building, called the Chrysalis, under construction in October 2022.
In a former farm field just outside the historic town of Janesville in south-central Wisconsin, a large concrete-and-steel building is taking shape. Dubbed the Chrysalis, the building will eventually house eight accelerator-based neutron generators, which start-up company SHINE Technologies will use to produce molybdenum-99. As the precursor to the medical radioisotope technetium-99m, Mo-99 is used in tens of millions of diagnostic procedures every year, primarily as a radioactive tracer.
At the heart of the Chrysalis will be the high-flux neutron generators, being supplied by SHINE’s sister company, Phoenix. The compact accelerators use a deuterium-tritium fusion process to produce neutrons, which in turn induce a subcritical fission reaction in an aqueous low-enriched uranium target (19.75 percent uranium-235) to produce Mo-99.
IBA Rhodotron TT300-HE (high energy) electron accelerator. (Photo: Business Wire)
Nuclear medicine company NorthStar Medical Radioisotopes announced that it has successfully produced molybdenum-99 at its recently completed accelerator production facility at its Beloit, Wis., campus. According to NorthStar, the event marks a major milestone in advancing the company’s proprietary electron accelerator technology for the non-uranium–based production of the critical medical radioisotope.
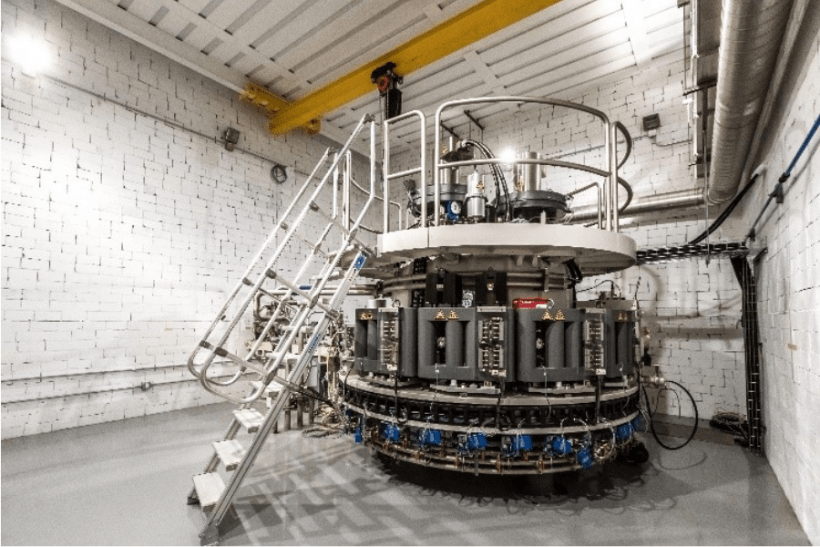
The electron accelerator that will be used for Mo-99 production at NorthStar’s newly completed facility in Wisconsin. (Photo: NNSA)
NorthStar Medical Radioisotopes has completed construction and all equipment installation at its new facility in Beloit, Wis., to produce the medical radioisotope molybdenum-99 without the use of high-enriched uranium, the Department of Energy’s National Nuclear Security Administration announced last week.
SHINE’s Mo-99 production facility under construction in Janesville, Wis. (Photo: SHINE)
SHINE Europe, a nascent subsidiary of Wisconsin-based SHINE Technologies, announced Wednesday that it has secured funding to begin designing an advanced medical isotopes facility in Veendam, the Netherlands. The new facility will use the same fusion-based neutron generator system SHINE is employing at its Janesville, Wis., facility to produce medical isotopes, including molybdenum-99, which is used in diagnostic imaging.
Darlington nuclear power plant. (Photo: OPG)
The Canadian Nuclear Safety Commission (CNSC) has amended Ontario Power Generation’s (OPG) operating license for its Darlington nuclear power station near Clarington, Ontario, allowing the company to produce the medical radioisotope molybdenum-99 using Darlington’s Unit 2 CANDU reactor. OPG subsidiary Laurentis Energy Partners, in conjunction with BWXT Medical, is leading the program to produce Mo-99 at Darlington.
SHINE Technologies’ headquarters building in Janesville, Wis. (Photo: SHINE)
The Department of Energy’s National Nuclear Security Administration has issued a cooperative agreement worth $35 million to SHINE Technologies, based in Janesville, Wis., to support the commercial production of molybdenum-99, a critical isotope used in more than 40,000 medical procedures in the United States each day, including the diagnosis of heart disease and cancer.
NorthStar’s RadioGenix system produces the medical radioisope Mo-99 without the use of uranium. (Photo: NorthStar)
NorthStar Medical Technologies of Beloit, Wis., will receive $37 million under two cooperative agreements with the National Nuclear Security Administration for the production of molybdenum-99 without the use of high-enriched uranium. Considered a critical medical radioisotope, Mo-99 is used in more than 40,000 medical procedures in the United States each day, including the diagnosis of heart disease and cancer.
A rendering of the SHINE medical isotope production facility planned for construction in Veendam, the Netherlands. (Image: Shine)
SHINE Medical Technologies plans to locate its European medical isotope production facility in the Netherlands after a yearlong search and a review of more than 50 proposals from sites across Europe. The company announced on May 20 that construction at the site should begin in 2023 with commercial production starting in late 2025.
SHINE executives, construction managers, and partners commemorate a construction milestone of the medical isotope production facility in March. (Photo: SHINE)
The Nuclear Regulatory Commission has approved a request by SHINE Medical Technologies for an exemption from regulations on how commercial grade equipment is defined, allowing the company to more easily procure components for the medical isotope production facility it is building in Janesville, Wis.
NorthStar is capable of producing Mo-99 using non-uranium-based processes. Photo: NorthStar Medical Radioisotopes
Completing a 5,700-mile journey from Belgium, two 24-ton particle accelerators were delivered to NorthStar Medical Radioisotopes’ facility in Beloit, Wis., on April 22, the Wisconsin State Journal reported. Photos and a video of the accelerators being received at the facility are included in the report.



.png)







