New uranium and fluorine detection method set to boost nuclear safeguards
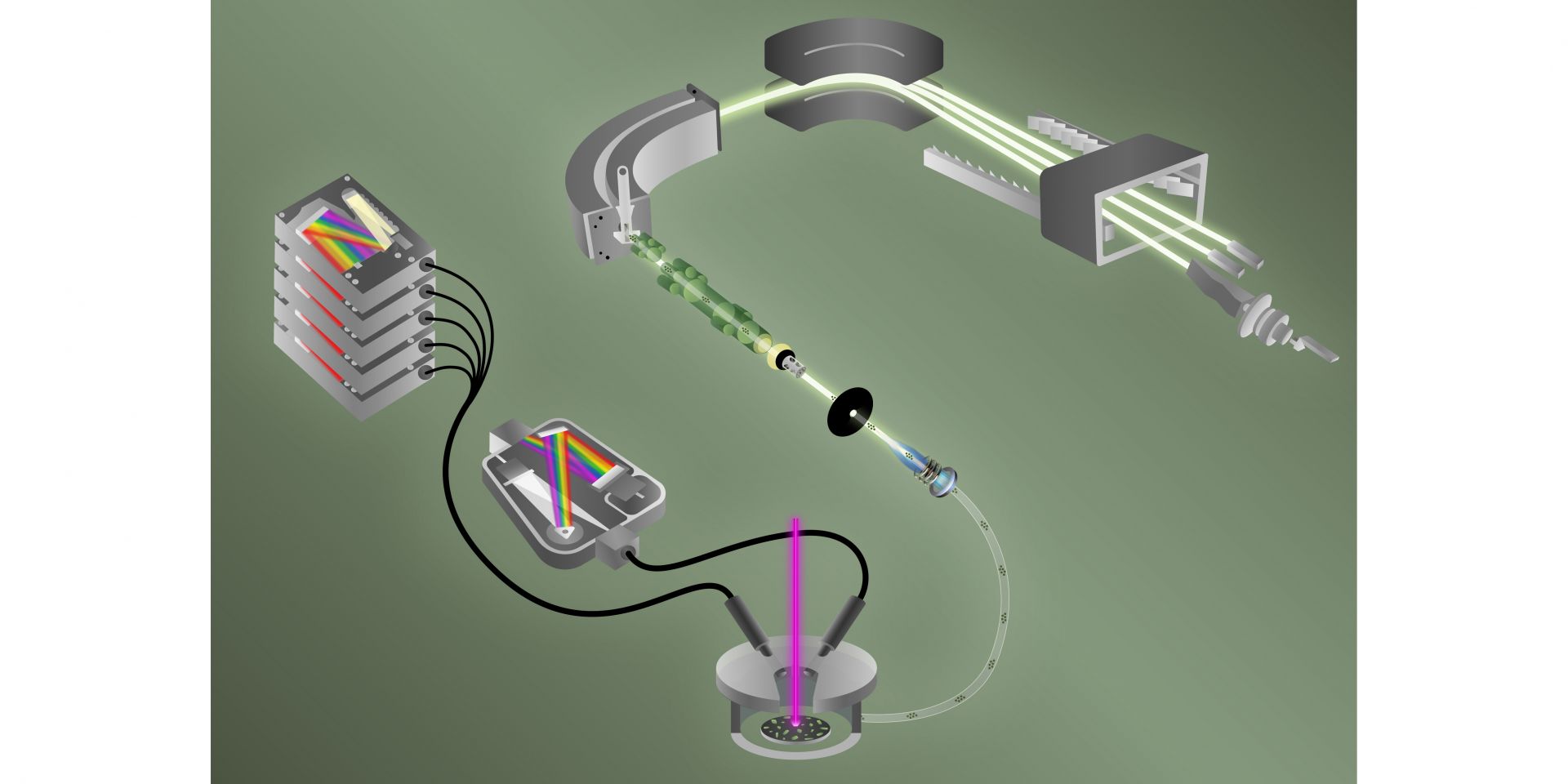
By combining two techniques, analytical chemists at Oak Ridge National Laboratory have for the first time simultaneously detected fluorine and different uranium isotopes in a single particle. Quickly detecting both elements together may help International Atomic Energy Agency inspectors determine if and when undisclosed enrichment has taken place. The findings, published in the Journal of the American Chemical Society, “push the limit” of how fast single particles can be characterized in terms of their chemical, elemental, and isotopic compositions, according to a September 26 news release from ORNL.

-3 2x1.jpg)