Neutron science reveals “fascinating chemistry” of molten fuel salts
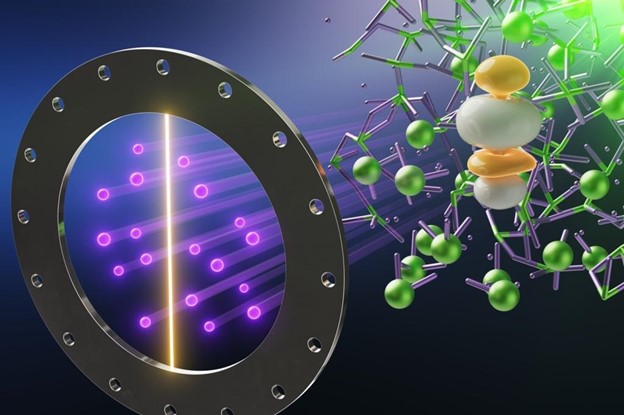
New research into the dynamics and structure of high-temperature liquid uranium trichloride (UCl3) salt—a potential fuel for molten salt reactors—has been published in the Journal of the American Chemical Society. A recent news release from Oak Ridge National Laboratory describes how researchers from ORNL, Argonne National Laboratory, and the University of South Carolina used ORNL’s Spallation Neutron Source (SNS) to document the unique chemistry of liquid UCl3 “for the first time.”

-3 2x1.jpg)